For a basic traceability matrix your columns will be.
Medical device design traceability matrix template.
Design control guidance for medical device manufacturers 1997.
1 the design requirements matrix or iovv i e inputs outputs verification and validation and 2 the risk traceability matrix.
Is there a documented traceability analysis or matrix linking product design requirements design specifications risks and controls and tests.
Risk analysis hazard traceability matrix template free 0 00 this is a downloadable template which applies to medical devices including in vitro diagnostic medical devices and active implantable medical devices.
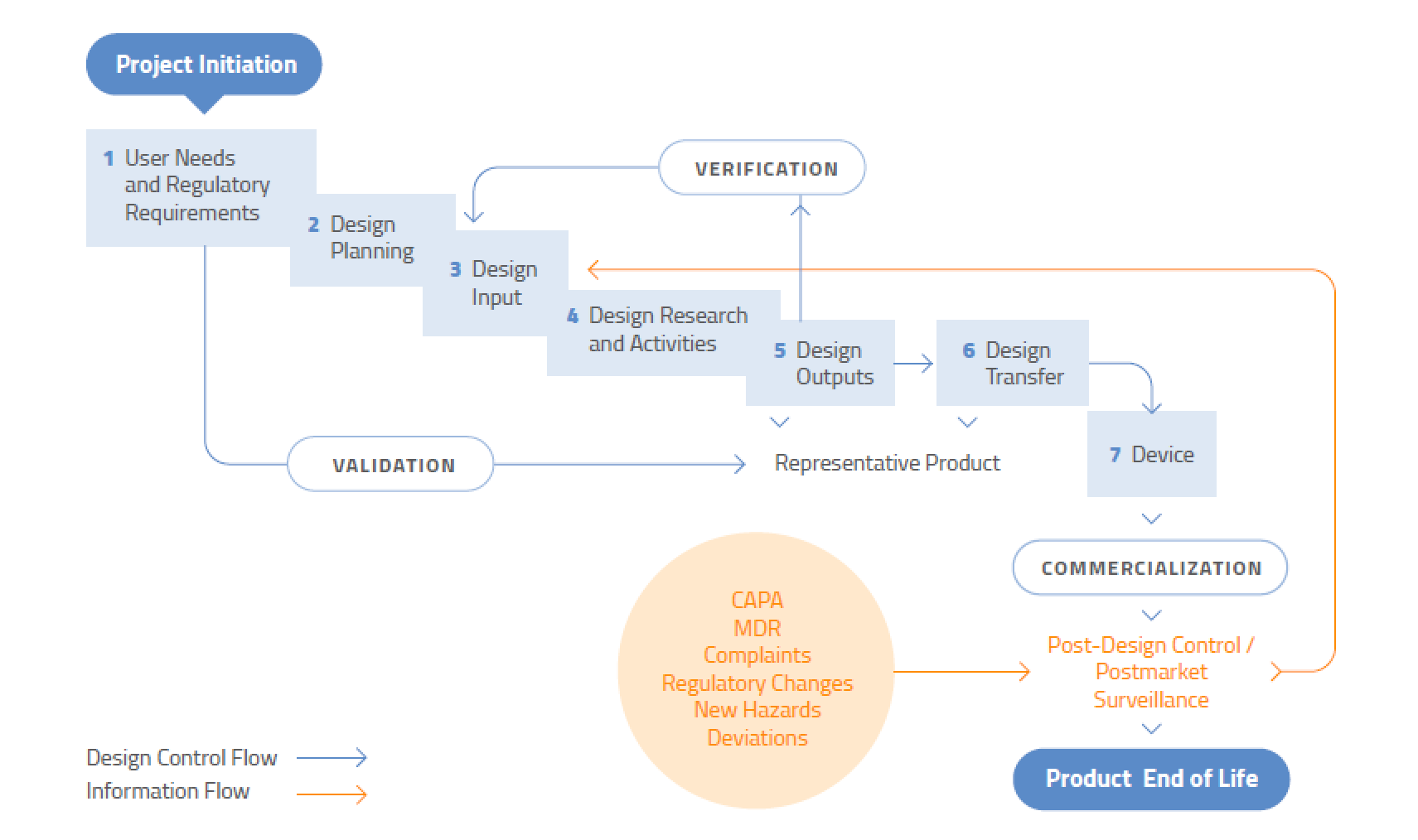
There are two common types of validation.
Confirmation of the device s design.
Medical device design validation.
The design requirements traceability matrix drtm is a combination of two documents that have been used for the past two decades by medical device manufacturers.
This traceability system is able to engrave logos datamatrix codes serial numbers or any other information required to identify a medical device.
Other requirements fda guidance documents.
Process validation uses objective evidence to make sure a process consistently produces the same result.
Manufacturers in the medical market and healthcare institutions are regulated by very strict.
You ll need to add a column for each of your artifacts.
Traceability of medical devices.
Create a traceability matrix template in excel.
Design verification is all about confirming by objective evidence that your device s design output meets its design input so that a manufacturer can say i made the product correctly in most cases comparing outputs to inputs shows that the device.
Laser marking on medical equipment enables you to get permanent marking resistant to sterilization processes.
Once you ve defined and gathered your documents you re ready to make your traceability matrix template.